
Containerized Modular Cleanroom
An All-in-one, Prefabricated Solution Engineered for Rapid Deployment, Flexible Expansion, and Sustainable Operations.


Struggling with building a pharmaceutical facility overseas?
You're not alone. Unpredictable timelines, budget overruns, immature supply chains, and uncertain regulatory compliance-these shouldn't stand in your way.
The Off-Site, On-Time Solution for Global Markets.
AksobioQ
The Off-Site, On-Time Solution for Global Markets.
The Off-Site, On-Time Solution for Global Markets.
How we cure the headaches:
De-risk your project With factory-built, pre-qualified modules shipped fully assembled, we eliminate on-site construction delays and accelerate approval and deployment.
Decouple from local supply chain limitations Our self-contained cleanroom modules operate independently-no reliance on local resources. Plug in and proceed.
Navigate regulatory complexity with confidence We design every system to meet target market regulations-so you stay compliant, anywhere in the world.
Learn More
De-risk your project With factory-built, pre-qualified modules shipped fully assembled, we eliminate on-site construction delays and accelerate approval and deployment.
Decouple from local supply chain limitations Our self-contained cleanroom modules operate independently-no reliance on local resources. Plug in and proceed.
Navigate regulatory complexity with confidence We design every system to meet target market regulations-so you stay compliant, anywhere in the world.


Why lock yourself into a rigid, expensive facility when your research-and funding-can change overnight?
In biotech, change is the only constant. Yet traditional construction/fit-out forces you to make long-term bets on layouts and capacity-just when you need flexibility the most.Don't just build-build smart.
The Smarter, Swifter Way to Build for Biotech.
AksobioQ
The Smarter, Swifter Way to Build for Biotech.
The Smarter, Swifter Way to Build for Biotech.
How we help you stay agile:
Change your layout as fast as your pipeline evolves: Adapt your facility quickly to new processes or scale up success-no demolition required.
Stay liquid, stay agile: Turn massive CapEx into nimble OpEx. Save your cash for the breakthroughs, not the brickwork.
Grow on your terms: Add capacity only when you need it. Avoid empty cleanrooms and wasted resources while staying ready for what's next.
Learn More
Change your layout as fast as your pipeline evolves: Adapt your facility quickly to new processes or scale up success-no demolition required.
Stay liquid, stay agile: Turn massive CapEx into nimble OpEx. Save your cash for the breakthroughs, not the brickwork.
Grow on your terms: Add capacity only when you need it. Avoid empty cleanrooms and wasted resources while staying ready for what's next.


Is your biopark offering yesterday's "space for rent" in a tomorrow world? Tenants don't want empty boxes.
They want speed, flexibility, and a partner-not a landlord. Stop competing on square meters; start competing on solutions.
The "Wow" Factor for World-Class Bioparks.
AksobioQ
The "Wow" Factor for World-Class Bioparks.
The "Wow" Factor for World-Class Bioparks.
Become the most desirable address in biotech:
Tailor-Made Solutions: We combine versatile functional modules to meet the unique needs of different enterprises, creating fully customized industrial spaces that truly fit.
Lease-Friendly & Flexible: Our modular facilities can be easily relocated and recycled. Adjust or return them based on lease changes-enjoy a zero-hassle, move-in-ready experience.
High-Efficiency Investment: Provide tenants with premium, differentiated value-added services without heavy investment. Stand out and attract more with minimal cost.
Learn More
Tailor-Made Solutions: We combine versatile functional modules to meet the unique needs of different enterprises, creating fully customized industrial spaces that truly fit.
Lease-Friendly & Flexible: Our modular facilities can be easily relocated and recycled. Adjust or return them based on lease changes-enjoy a zero-hassle, move-in-ready experience.
High-Efficiency Investment: Provide tenants with premium, differentiated value-added services without heavy investment. Stand out and attract more with minimal cost.


Need a full-grade environmental-compliant lab-deployed anywhere, anytime?
When outbreaks hit or disaster strikes, you can't wait for builders to pour concrete. You need a clinical-grade environment that lands like a superhero-now.
Mission-Impossible Cleanroom Facilities. Possible.
AksobioQ
Mission-Impossible Cleanroom Facilities. Possible.
Mission-Impossible Cleanroom Facilities. Possible.
How we deploy hope at the speed of need:
Deployment-Ready: In uncontrolled settings-battlefields, remote areas, epidemic zones-we quickly build compliant medical spaces. Break through the "last mile" of critical care and bring life-saving technology right where it's needed.
Ensure compliance in uncontrolled settings: Our units are engineered to meet stringent international standards right out of the box, ensuring quality and safety wherever you operate.
Adapt to any mission profile: Customize the configuration to your exact needs-be it a diagnostic lab, mobile surgery unit, or vaccination center. Get a purpose-built facility without the build time.
Learn More
Deployment-Ready: In uncontrolled settings-battlefields, remote areas, epidemic zones-we quickly build compliant medical spaces. Break through the "last mile" of critical care and bring life-saving technology right where it's needed.
Ensure compliance in uncontrolled settings: Our units are engineered to meet stringent international standards right out of the box, ensuring quality and safety wherever you operate.
Adapt to any mission profile: Customize the configuration to your exact needs-be it a diagnostic lab, mobile surgery unit, or vaccination center. Get a purpose-built facility without the build time.
AksobioQ: Designed for Speed, Compliance, and Seamless Integration.
Fast-Track Your Cell Therapy from Lab to Clinic
Fast-Track Your Cell Therapy from Lab to Clinic
End-to-End Expertise: Partnering with industry leaders, we deliver full-scope solutions covering compliant facilities, cutting-edge processes, and gold-standard technologies. Simplify the journey from R&D to clinical application and boost both the efficiency and success rate of your cell therapy products.
Treat Without Interruption: Our modular sterile units-pre-assembled and container-based-allow rapid on-site deployment within hospital settings. Minimize construction impact, maintain continuous patient care, ensure safety, and accelerate clinical translation-all with zero downtime.
R&D and Production Solutions
Provides stable ISO 5-7 (B+A) core environments, ISO 8 (C) preparation zones.
Compliant with ISO classifications, adhering to NMPA or FDA GMP regulations.
Plug-and-Play.
Compliant with ISO classifications, adhering to NMPA or FDA GMP regulations.
Plug-and-Play.
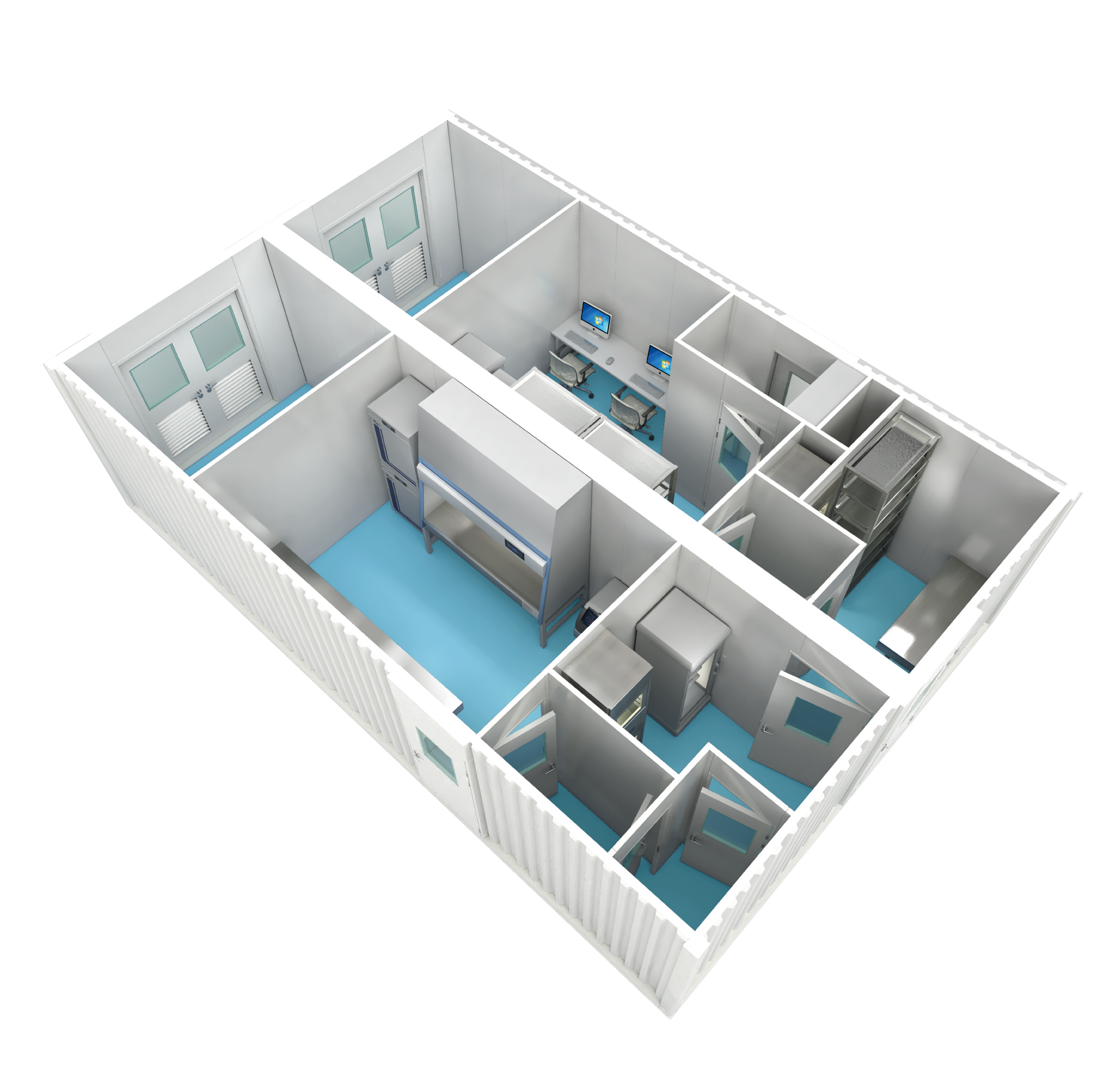
Stable Clean Environments
(GMP B+A Grade)
Compact Footprint, Low Energy Consumption
(Minimum size: 3m × 9m)
Plug-and-Play, Easily Scalable
(On-Site Installation Within 48 Hours)
Intelligent, Integrated, Informational
(Full-Stack Intelligence Management Platform)
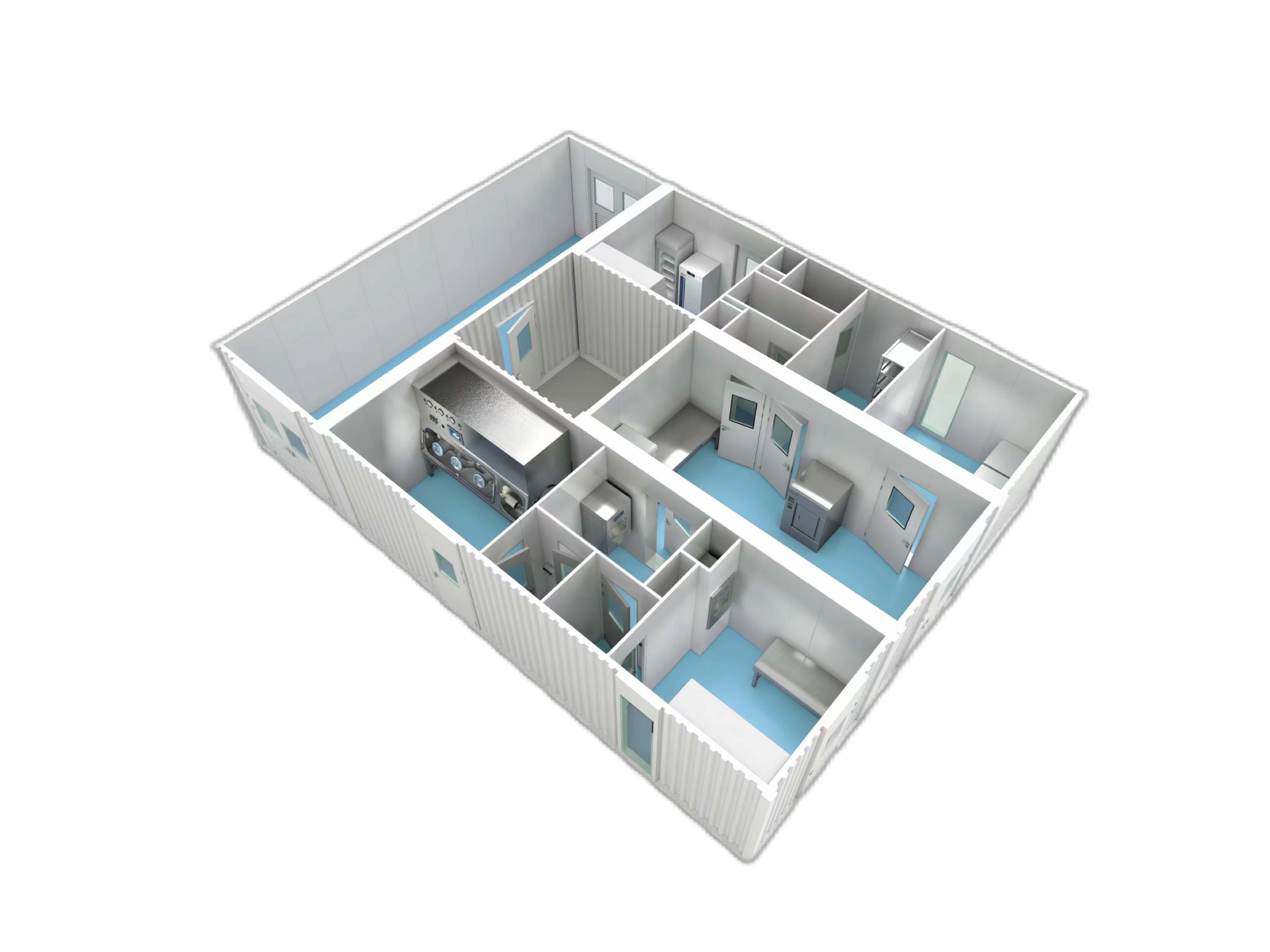
Quality Control Solutions
Includes aseptic testing zones, P2 positive control areas, cell culture operation zones, microbial limit testing zones, cleaning zones, and more.
Compliant with ISO classifications, adhering to NMPA or FDA GMP regulations.
Compliant with ISO classifications, adhering to NMPA or FDA GMP regulations.
AksobioQ Smart Cube
Containerized GMP Cleanrooms - Rapid Deployment/Flexible Iteration/Fully Validated Accelerating Biocompliance and Innovation.


AksobioQ
Choose the Perfect Fit. Deploy with Confidence.
Choose the Perfect Fit. Deploy with Confidence.
Rapid Deployment: Our modular cleanrooms are factory-built, delivered ready to install, and operational in record time. Simply assemble on-site-and start production. No delays. No surprises.
Expert Team: Our company boasts a team with nearly 30 years of industry expertise, capable of precisely capturing client needs to design 100% aligned cleanroom solutions. Precision-engineered for a 100% fit.
End-to-End Service Support: From blueprint to startup, our professionals oversee every step. We ensure not only a fast build but also long-term, reliable performance. Your project is safe with us.
Expert Team: Our company boasts a team with nearly 30 years of industry expertise, capable of precisely capturing client needs to design 100% aligned cleanroom solutions. Precision-engineered for a 100% fit.
End-to-End Service Support: From blueprint to startup, our professionals oversee every step. We ensure not only a fast build but also long-term, reliable performance. Your project is safe with us.
AksobioQ Standardized Solutions
Need a cleanroom that fits your budget and timeline? Go standard. Go fast.
Rent or Buy, Rapid Installation, Ready Day One.
Single Module: Provides basic cleanroom environments, ideal for pairing with physicochemical labs.
Dual Module: Features a clean zone + dedicated storage and data recording areas. Fully self-sufficient.
Multi-Module: Includes multiple clean spaces designed to support more complex R&D and testing workflows.
Rent or Buy, Rapid Installation, Ready Day One.
Single Module: Provides basic cleanroom environments, ideal for pairing with physicochemical labs.
Dual Module: Features a clean zone + dedicated storage and data recording areas. Fully self-sufficient.
Multi-Module: Includes multiple clean spaces designed to support more complex R&D and testing workflows.
AksobioQ Customized Solutions
hen standard isn't enough-engineer what's possible.If your processes are complex and demand more than an off-the-shelf cleanroom, choose a solution designed around your unique workflow.
100% Process-Matched. Maximized Space Efficiency.
We tailor every detail to your technical requirements and site conditions-delivering a cleanroom that fits like it was born there.
Future-Ready Flexibility.
Your custom facility won't just meet today's needs-it's designed to adapt and expand as your processes evolve and your business grows.
100% Process-Matched. Maximized Space Efficiency.
We tailor every detail to your technical requirements and site conditions-delivering a cleanroom that fits like it was born there.
Future-Ready Flexibility.
Your custom facility won't just meet today's needs-it's designed to adapt and expand as your processes evolve and your business grows.

Infusing Digital Vitality into AksobioQ
Medointec Laboratory Intelligence Platform
Equipment and Environment Monitoring, HVAC System Oversight, Safety Assurance, Data Dashboard Visualization, Traceable Record Management, User Permission Controls
RDtoRA Electronic Lab Notebook (ELN)
Project Management, Experiment Management, Design of Experiments (DOE), Instrument/Material/Reagent Management, Lab Instrument/Engineering Equipment Connectivity

Digital Intelligence Management

Cloud/Local Deployment

Holographic Recording
and Analysis
and Analysis

Data Integrity
Multi-Interface IoT

Scenario-Fusion Customization
Presents lab building/equipment layouts and operational status in 3D visualization, supporting multi-angle zooming and detailed views for enhanced intuitive oversight.
Supports both local installation and web-based remote access for digitized equipment management and real-time information monitoring, ensuring timely issue resolution.
Displays real-time and historical data on environments and equipment intuitively, with in-depth analysis focusing on key metrics to provide precise decision-making support.
Integrates audit trails, flexible permission management, approval workflows, and electronic signatures to comprehensively safeguard the authenticity, accuracy, and validity of experimental data.
Connects various instruments, devices, robots, and data systems for automated result transmission, integrating with other systems or applications to prevent lab information silos.
Customizes data recording templates based on specific experiment/process workflows, aligning closely with real-world research and production applications.
AksobioQ - Seamless Delivery from Empty Space to Smart Facility
Your Journey to A Fully Operational Cleanroom-made Simple, Structured, and Stress-free.
Your Journey to A Fully Operational Cleanroom-made Simple, Structured, and Stress-free.
You must go through the following steps:

Site Assessment & Planning

Requirements Confirmation

Contract Signing

Site Preparation

Rapid Installation & Deployment

Validation & Training
Our expert team conducts a thorough evaluation of your space-including infrastructure, environment, and resources-to deliver an optimized layout and truly tailored solutions.
Starting from your specific needs, we integrate site conditions to finalize professional clean facility designs, ensuring perfect alignment with expectations,confirmed via drawings.
Upon verifying all reasonable requirements are met, both parties sign the contract; we commit to full oversight of manufacturing, guaranteeing quality and on-time delivery.
We offer diverse logistics services to transport containerized cleanrooms to any specified location; you simply ensure the site meets installation prerequisites.
Pre-built modules mean minimal on-site work and maximum speed. We ensure everything performs exactly as promised.
We test everything thoroughly and train your team-so you're ready to operate confidently and pass inspections with ease.
Zhejiang Medointec co., Ltd.
30 Years of GMP Design and Quality Management Expertise in the Pharmaceutical Industry Safeguarding Biopharmaceutical Innovation, Accelerating Enterprise Commercialization









 WeChat
WeChat